Nature Subsidiary: CAR-T cell expansion efficiency is expected to increase 10 times
January 23, 2018 Source: Academic Jingwei
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Last year was the first year of CAR-T therapy – a breakthrough therapy that allowed one cancer patient to survive, turning a “death judgment†into a miracle of life. The advent of two CAR-T therapies has also opened a new era of cancer treatment.
   
  
However, the approval of the listing is not the end of the development of such treatments. Researchers are well aware that there are still many bottlenecks in this type of therapy. From a process perspective, researchers need to isolate immune T cells from patients and send them to the laboratory to introduce receptors for cancer cell surface antigens. Subsequently, the researchers expanded the number of T cells that introduced the new gene into a million-level quantity and then returned it to the patient to treat cancer.
The rate limiting step here is that the in vitro amplification step of T cells is time consuming and labor intensive. In humans, expansion of T cells requires the assistance of antigen-presenting cells (APCs). Such cells will multi-step stimulation of T cells at the right time. However, in vitro expansion, scientists are not able to mimic the activation of T cells by APC cells.

â–²Professor David J Mooney of this study (Source: Harvard University)
This phenomenon is expected to be completely changed! Recently, "Nature Biotechnology" published in the "Nature" magazine published a study that is inspiring for everyone who cares about cell therapy: Professor David J Mooney from Harvard University's Wyss Institute and his team developed a use A scaffold made of biomaterial. It mimics the function of APC in vitro and makes T cell expansion more efficient. If it can be further applied to the clinic, we will hope to shorten the manufacturing process of CAR-T therapy and let it come to patients who suffer from acute blood cancer as soon as possible for treatment.
To make the stent, the researchers first filled the porous silicon rod-like material with interleukin 2 (IL-2), a class of factors produced by APC that prolongs the lifespan of T cells. The researchers then applied a lipid bilayer to the outside of these silicon rods to mimic the cell membrane of APC. Later, they added a variety of antibodies that activate T cells. In the culture dish, these small rods can automatically and automatically form a scaffold structure, allowing T cells to move and expand freely in the scaffold.
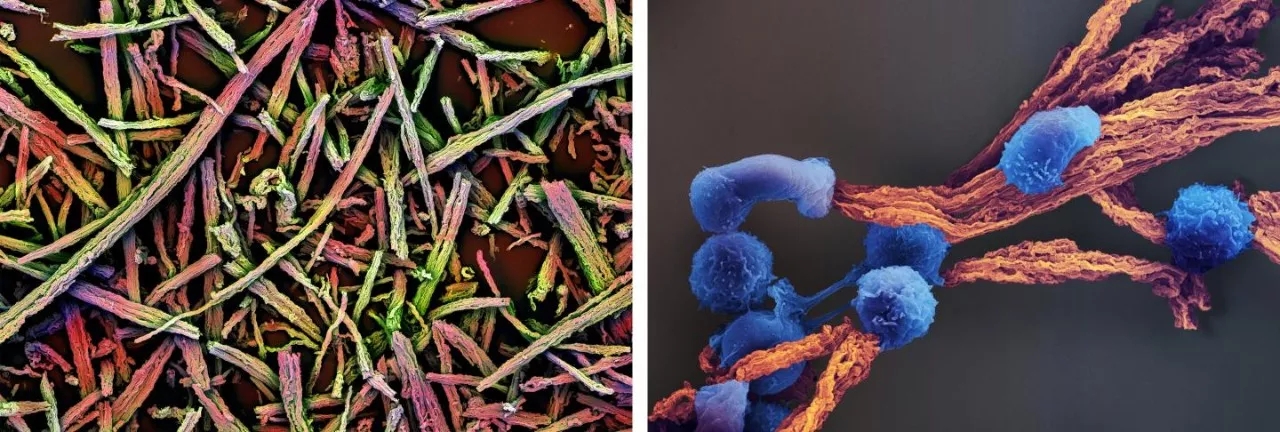
â–² Electron microscopy of the scaffold (left), and T cells attached to the scaffold (blue on the right) (Source: Wyss Institute at Harvard University)
This seemingly simple design has achieved good results. These mock APC scaffolds are surprisingly efficient compared to current conventional in vitro amplification techniques. "After one use, the mock APC scaffold can increase the proliferation of primary T cells in mice and humans by 2-10 fold!" David Zhang, the second author and graduate student of the study, said: "Another benefit of this stent." It allows us to fine-tune the subpopulation of T cells to produce the desired immune response. In the future, this will increase the functionality of T cells."
Subsequently, the researchers also tested whether this scaffold can contribute to the expansion of CAR-T cells. The results indicate that such scaffolds can produce more CAR-T cells than conventional amplification protocols. In terms of efficacy, these fast-producing T cells can also maintain good cancer cell killing effect. "We show that these CAR-T cells amplified with a mock APC scaffold can help treat human lymphoma in a mouse model," said Dr. Alexander Cheung, the lead author of the study.
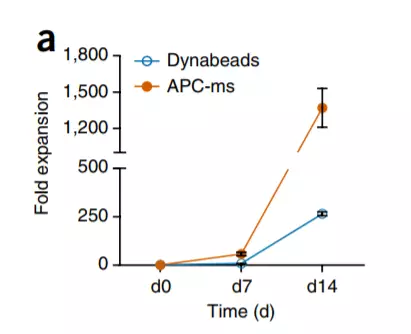
â–² Simulated APC scaffold (APC-ms) is amazingly efficient in cell expansion (Source: Nature Biotechnology)
"Our approach closely mimics the stimulation of primary T cells by APCs and also mimics the mechanism by which APCs enhance T cell survival by releasing soluble factors," commented Professor Mooney of the study. On the top, we can amplify cells faster and better. By adjusting the ratio of lipids, new drugs, and other factors, we can create a very diverse platform to amplify specific T cell populations for CAR- T and other treatments."
We expect this technology to be applied clinically as soon as possible, bringing faster and better treatment to patients.
Reference materials:
[1] Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells
[2] Fast-tracking T cell therapies with immune-mimicking biomaterials
Dried Beet,Dried Red Beet,Dried Beet Root,Dehydrated Red Beet, Beet Root Powder
Jiangsu Tiankang Food Co., Ltd. , https://www.tiankangfood.com