Treatment of migraine, the key clinical phase of new drug success
February 07, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Pharmaceutical company Allergan today announced the positive results of its third phase clinical trial, ACHIEVE I, in the study of new drug ubrogepant for migraine, the first of two key phase 3 clinical trials. The results showed that the safety and tolerability of oral administration of ubrogepant 50 mg or 100 mg showed positive results in a single migraine attack in adults compared with placebo.

Migraine is a chronic condition with neurological symptoms such as headache, sensitivity to light, sound, and nausea. These symptoms are usually incurable. Migraine is very common, affecting about one in seven people around the world and is associated with significant disability, leading to social and economic burdens. Current care standards for acute treatment of migraine are not optimal for many patients, and there are limitations such as limited efficacy, poor tolerance, or contraindications. Patients may experience uncontrolled migraine repeatedly, leading to increased drug overuse and increased risk of disease progression. Compared with current standards of care, new migraine treatments are needed to increase effectiveness, reduce risk, and benefit more patients.
Ubrogepant is a novel oral calcitonin gene-related peptide (CGRP) receptor antagonist currently developed for the treatment of acute migraine. CGRP and its receptor are expressed in the nervous system region associated with the pathophysiology of migraine. CGRP receptor antagonism is a new mechanism of action in the acute treatment of migraine, which is significantly different from the existing triptans (serotonin 1B / 1D agonist) and opioids.
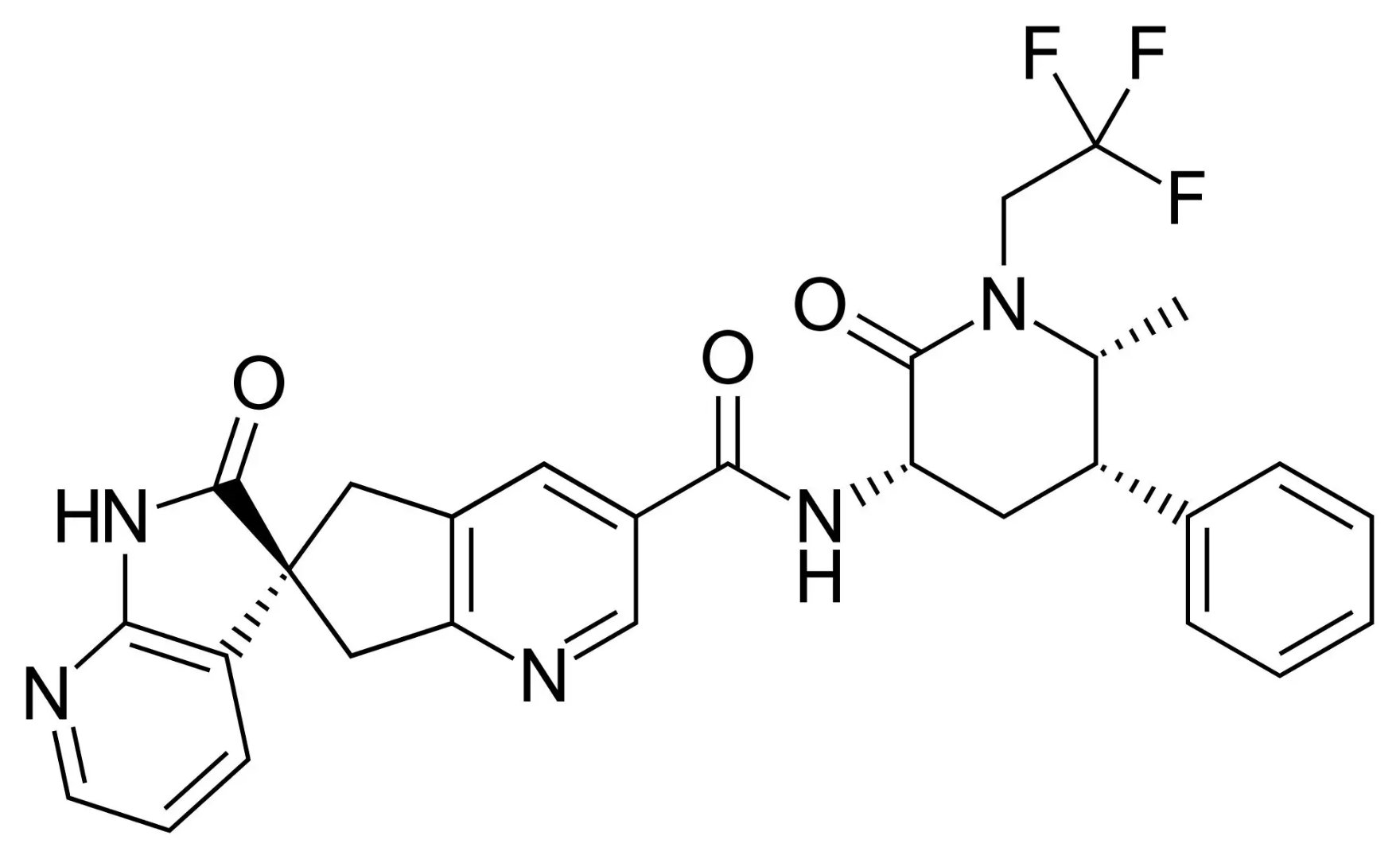
â–²Ubrogepant's molecular structure (Source: Wikipedia)
The ACHIEVE I trial was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate two doses of ubrogepant (50 mg and 100 mg) compared to placebo in a single migraine attack Effectiveness, safety and tolerability. The study enrolled 1327 adult American patients with moderate to severe migraine and were randomized to three groups: placebo, 50 mg, and 100 mg. The study showed that the percentage of pain relief achieved by the two doses at 2 hours after initiation of treatment was significantly higher than in the placebo group (50 mg vs placebo, p = 0.0023, 100 mg vs placebo, p = 0.0003), At the same time, the percentage of the most disturbing symptoms associated with migraine (such as photophobia, phobia, or nausea) in both dose groups was significantly higher than in the placebo group (50 mg vs placebo, p = 0.0023). , 100 mg vs placebo, p = 0.0023). Ubrogepant is well tolerated and similar to placebo. The most common adverse events are nausea, lethargy, and dry mouth.

â–² Dr. David Nicholson, Executive Vice President and Chief R&D Officer of Allergan (Source: Allergan's official website)
Dr. David Nicholson, Executive Vice President and Chief Development Officer of Allergan, said: "We are pleased with the positive results of the ACHIEVE I study, which supports the effectiveness, safety and tolerability of ubrogepant, and we believe that the oral CGRP receptor antagonist ubrogepant will One of the treatments for adult migraine. Allergan is committed to exploring, developing and launching therapies that treat these annoying diseases to meet the needs of patients."
The main investigator of the project, Dr. Richard B. Lipton, deputy director of the Department of Neurology at the Albert Einstein College of Medicine and deputy director of the Department of Neurology, said: "Although the incidence of migraine is high and there are Several treatment options are available, but the disease is still not fully diagnosed and treated, and compliance with current treatment standards is poor, and we still need new treatments to improve the current situation. The results are important for the research and development of therapies for the treatment of migraine patients."
Other results of this study will be presented at the upcoming 2018 Science Conference. The results of another phase 3 clinical trial, ACHIEVE II, are expected to be completed in the first half of 2018. Allergan plans to submit a new drug application (NDA) to the FDA in 2019.
We are very happy to see this gratifying progress in the field of migraine, and we look forward to more effective, low-risk new drugs to alleviate the suffering of patients.
Reference materials:
[1] Allergan Announces Positive Top Line Phase 3 Results for Ubrogepant - an Oral CGRP Receptor Antagonist for the Acute Treatment of Migraine (Allergan's official website)
[2] Allergan Shows Off Phase III Migraine Data
Collapsible Foot Spa,Foot Spa Bath Massager,Foot Bath Spa Massager,Foot Soak Tub
Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootbath.com