Metastatic prostate cancer has new drugs! Greatly improve absorption efficiency
May 25, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Sun Pharmaceutical Industries and Churchill Pharmaceuticals jointly announced that the US FDA has approved the application for the innovative drug Yonsa. Yonsa is an innovative pharmaceutical formula for abiraterone acetate that will be used in combination with methylprednisolone for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC).

Prostate cancer is one of the most common cancers in men. Prostate cancer usually grows slowly and is initially confined to the prostate, but certain types of prostate cancer are highly aggressive and can be transferred quickly. Hormone therapy is one of the common treatments for prostate cancer that has metastasized. Because prostate cancer cells rely on androgen to help them proliferate, cutting off the supply of androgen can cause tumor cells to die or delay their growth.
Abiraterone acetate is a hormone therapy that inhibits androgen synthesis. Abiraterone acetate is converted into abiraterone in vivo, which is an inhibitor of CYP17 enzyme, which is expressed in testis, adrenal gland and prostate cancer tissues and is an essential protease for androgen biosynthesis.
Yonsa is unique in its use of Churchill's SoluMatrix Fine Particle Technology, an innovative pharmaceutical manufacturing technology. This technique grinds the drug particles into powders less than 1 micron in diameter while preventing the powder from re-aggregating. Drug particles produced using this technique are 10-200 times smaller than conventional drug particles, which greatly increases the solubility of oral drugs and the efficiency of absorption by the body.
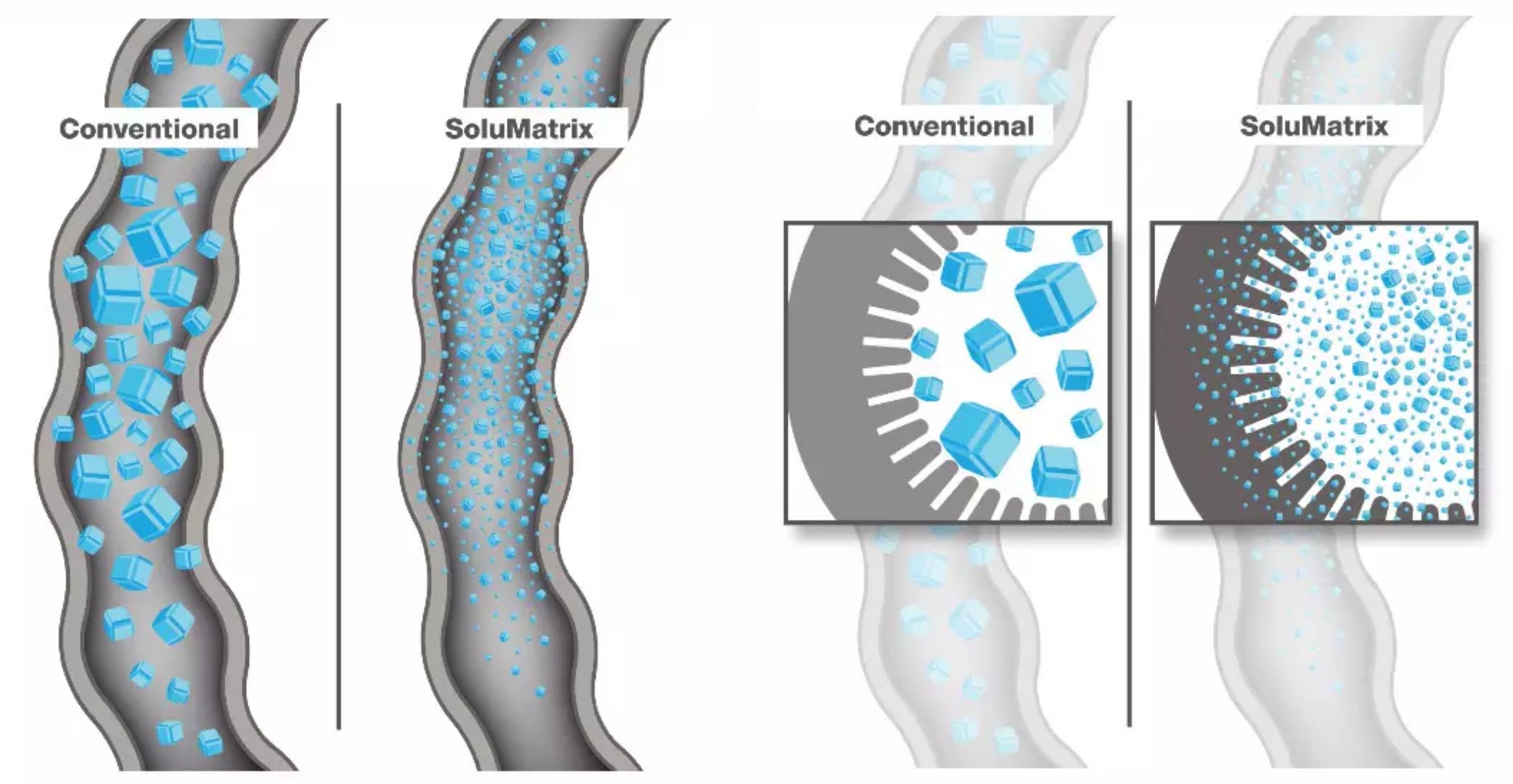
â–²Using SoluMatrix Fine Particle Technology technology can greatly increase the solubility and absorption efficiency of drugs
(Source: Churchill Pharmaceuticals official website)
In the closed open-label, randomized phase 2 clinical trial called STARR, a combination of 500 mg of Yonsa and methylprednisolone daily with 1000 mg of Zytiga (traditional formulation of abiraterone acetate) and prednisone (prednisone) Compared to combination therapy, therapeutic equivalency is achieved in reducing androgen levels in the patient's serum.
“We are pleased to be adding Yonsa to our growing oncology drug products. Sun Pharma will continue to focus on making it easier for patients to access innovative anticancer therapies,†said Abhay Gandhi, CEO of Sun Pharma North America.
Reference materials:
[1] Sun Pharma Announces USFDA Approval of YONSA® (abiraterone acetate) To Treat Metastatic Castration-Resistant Prostate Cancer In Combination With Methylprednisolone
[2] Churchill Pharmaceuticals official website
[3] Churchill Pharmaceuticals Announces NDA Filing Acceptance for YONSATM by the US FDA
Frozen Bonito Tuna Skipjack Products
Zhoushan City Shuangying Aquatic Products Co., Ltd.  , https://www.shuangying-aquatic.com