Release date: 2016-12-29

New Pulse Medical Founder Qi Qifeng
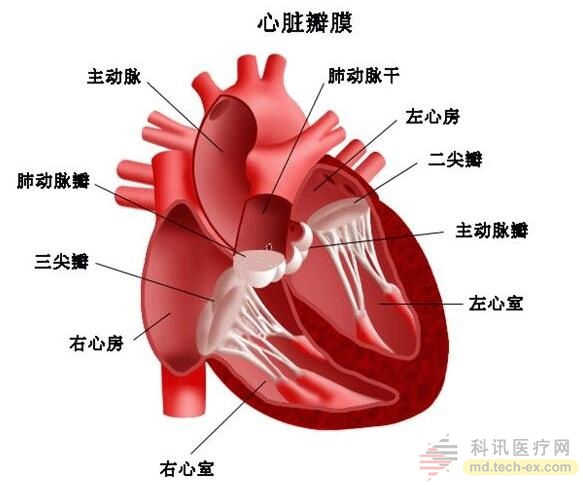
Compared to the aorta, we are afraid of being stranger to the mitral valve. The mitral valve, also known as the sacral flap, acts as a "one-way valve" between the left atrium and the left ventricle, ensuring that blood circulation must flow from the left atrium to the left ventricle and through a certain blood flow. When the left ventricle contracts, the blood in the chamber is squeezed and the blood hits the valve. The mitral valve closes and the blood does not flow into the left atrium.
According to statistics, there are about 26.8 million patients with mitral regurgitation in China. With the aging of the population, the mitral regurgitation is growing at a rate of 3% per year. The number of newly added patients is not less than 800,000. About 60 million cases will be reached in 2050.
In the past two years, the industry has become more and more concerned about the mitral valve, which has become a new wave in the valve industry. Shanghai New Pulse Medical Technology Co., Ltd. (hereinafter referred to as “New Pulse Medicalâ€) located in Shanghai International Medical Park is committed to the research and development and industrialization of minimally invasive/interventional artificial heart valve system at the international advanced level. It is the only one in China that has independent research and development. The company of the transcatheter mitral valve system.
Interventional heart valves are an inevitable trend
Mitral regurgitation is mainly caused by mitral regurgitation. Physiological changes in the mitral valve aggravate left atrial load and left ventricular diastolic load, while elevated left atrial pressure can cause elevation of pulmonary vein and pulmonary capillary pressure, followed by dilation and congestion, while left ventricular diastolic volume load increases. The continued development of mitral regurgitation will cause severe heart failure, which will seriously reduce the quality of life of patients and increase the incidence of sudden death.
"In China, the incidence of mitral valve is higher than that of the aortic valve, which is 1.5-2 times. Different from the current aortic valve, it can be used for thoracotomy or minimally invasive intervention to achieve good therapeutic effect. The flap has only 2% effective treatment rate," said Qi Qifeng, chairman of New Pulse Medical, in an interview with Medical Valley.
The following are the advantages and disadvantages of surgical repair, surgical bioprosthetic replacement and interventional valve/valvular repair.

From this we can see that minimally invasive surgery as a revolutionary treatment, alternative surgery for thoracotomy has been a development trend, with the official entry of clinical products into the clinical, it is expected that clinical demand will show explosive growth in the next few years.
As we all know, aortic valve intervention technology has been relatively mature, and nearly 300,000 cases have been applied in the world. However, the interventional treatment of the mitral valve is much earlier than the aortic valve intervention. It is because the mitral anatomy is difficult for all valve crowns and related products. Therefore, the aortic valve intervention product is earlier than this. Enter the clinical application. Qi Qifeng said that above the aortic valve is a tube (aortic arch), the path and fixation method is relatively easy to solve, but the mitral valve annulus is not easy to fix due to complicated shape, easy to cause valve leakage, and easy Blocks the passage between the left ventricle and the aorta. Over the years, international exploration has been in the process of repeated design improvements and animal experiments.
With the maturity of aortic valve interventional therapy technology, the rapid development of CT, angiography, ultrasound and other auxiliary imaging technologies, a large amount of technical experience accumulated in this process will promote the further development of mitral valve interventional therapy.
In 2012, mitral valve minimally invasive/interventional therapy completed the first clinical trial. By 2014, many foreign companies had entered the field to develop related products. In 2015, Medtronic, Edward, and Abbott each acquired a company with minimally invasive/interventional treatment in the field of mitral valve. The three giants in the field of international medical device heart valves have officially entered the field of minimally invasive mitral/interventional therapy. Both medical device companies and mainstream doctors believe that mitral valve minimally invasive/interventional therapy is likely to become the standard of treatment in the next five to ten years.
Development of domestic artificial heart valves
According to Qi Qifeng, in the 1970s and 1980s, because the pioneers of cardiac surgery in China have done a lot of basic and applied research in the field of heart valves, they are also in a relatively leading position in the world, but due to lack of relevant policies and financial support. The backwardness of the domestic medical device industry, many research results have not been able to get the final conversion, or even lost the inheritance. In the early 1990s, China fell across the board in this field. It was completely monopolized by the international companies such as Medtronic, Edward, St. Jude, and it was not until 2000 that the emergence of domestic companies re-emerged. Therefore, as of now there are no domestically produced open thoracic flaps and interventional valves in China, Chinese patients can only pay for high prices to use imported products.
At the same time, due to the influence of foot-and-mouth disease and mad cow disease, China General Administration of Inspection and Quarantine strictly controls the treatment of bovine pericardial valve into China. At present, more than 90% of the Chinese market is mechanical, with few biological valves, most of which are pig pericardial valves. However, in fact, the bovine pericardial valve is the closest state of the human valve. This is because the bovine pericardium is closest to the laminar flow of the human body, and it is not easy to induce thrombosis, and the incidence of complications is low.
“In 2010, with the rise of innovation consciousness, the changes in China's health market and the increase of risk funds, the domestic heart valve field has ushered in new developments. For example, Xinjit's open chest valve technology uses Australian imported cattle pericardium. It is in line with Edwards. The aortic interventional valves of Qiming Medical, Wei Chuang Medical, Jiecheng Medical and other companies have completed clinical trials. It is expected to be approved by CFDA within two years and officially launched. The domestic aortic valve intervention field will be rapid. Mature," said Qi Qifeng.
In the field of minimally invasive/interventional treatment of mitral valve, Qi Qifeng believes that although domestic research and development is behind some time in Europe and America, this may not be a bad thing. “European and American R&D experience can make us less detours. At the same time, mitral minimally invasive/interventional therapy is an innovative product and an innovative technology. The recognition of European and American clinicians also contributes to the development of the domestic market. In terms of medical treatment, although it is slightly later than the research and development of foreign products, the foreign similar products are currently in the stage of obtaining CE and FDA certification. When entering China, they also need to go through the same CFDA-related certification and approval process as New Pulse Medical. It seems that New Pulse Medical's products are able to take the lead."
Grasping the wind, independent innovation
It is reported that New Pulse Medical's mitral valve minimally invasive valve system (Mi-thos), based on the principle of catheter valve surgery has been widely used, with intervention, no surgical suture, no extracorporeal circulation, etc., can make heart valve The replacement surgery procedure is simplified, the treatment risk is greatly reduced, and the success rate is significantly improved.
"A small incision through the apex of the apex, the product automatically bounces off the catheter into the mitral region. The thoracotomy usually takes four to five hours, and we only need one or two hours." Qi Qifeng said. At present, the product is in the stage of animal experiment, has obtained more than 40 patents, and is expected to be available in about 6 years.
At present, the core team of New Pulse Medical has more than 15 years of industry experience. The cooperative domestic hospitals and universities include Fuwai Hospital, Zhongshan Hospital, Xijing Hospital, Sichuan University, Zhejiang University, Tsinghua University, etc. in terms of raw materials and product manufacturing. Keeping up with international standards, international partners are companies with long-term relationships with well-known companies such as Medtronic and Edward.
In August of this year, New Pulse Medical's 13th Five-Year Key Project “Biomedical Materials R&D and Tissue Organ Repair Replacement†was approved by the Ministry of Science and Technology and received financial support from the Ministry of Science and Technology and the Ministry of Finance, and entered the special approval channel for national medical devices. On November 28th, New Pulse Medical was awarded the third place in the finals of the 5th China Innovation and Entrepreneurship Competition (Biomedical Industry).
While improving product development, New Pulse Medical is also actively involved in the field of raw material supply. Qi Qifeng said that because the domestic animal husbandry industry did not establish a complete traceability system, the cattle pericardium materials need to rely on imports, resulting in high costs. In order to reduce costs and obtain high-quality raw materials, and to expand new market opportunities, New Pulse Medical has begun to try to cooperate with large-scale farms to explore the establishment of an all-natural and pollution-free traceability system from breeding to slaughter in Sichuan. Good results will be achieved during the year.
"I hope that through the unremitting efforts of New Pulse Medical, we will be able to bring more quality and cheap products to our patients at an early date." Qi Qifeng looked forward with confidence.
Source: Medical Valley
Salted Black Bean With Salted Ginger
Salted Edamame Beans,Low Salt Beans,Low Sodium Pinto Bean,Salted Soy Bean
jiangmen city hongsing food co., ltd. , https://www.jmhongsing.com