Release date: 2017-11-15
For the treatment of patients with ischemic stroke, the speed of removal of blood clots is a key indicator. If the rescue is not timely and the treatment is slow, it is likely to affect the healing effect. However, traditional chemical drugs have a slower thrombolytic effect and cannot meet the speed requirement for the treatment of patients with acute ischemic stroke, which is why many patients with stroke have died.
From the current research, the combination of chemical thrombolysis and mechanical thrombectomy is a better treatment. The so-called mechanical thrombectomy is to use the bolting stent to remove the embolus that blocks the blood vessel. At present, there are few companies in China that are engaged in such research and development. The market for mechanical bolting equipment is mainly based on the products of foreign companies such as Medtronic and Johnson & Johnson.
The introduction of Perflow is an Israeli neurology medical equipment company. This year, it has completed a new round of financing jointly invested by China-Jilin Fund, Jingxin Pharmaceutical and SCI.
Founded in 2010, Perflow designs and develops advanced medical devices for the treatment of strokes and other intracranial disorders through its patented technology platform CEREBRAL NET® weaving design technology. Its founder, Gilad, is a woven and bioengineering design expert who has researched and developed products for many well-known companies such as MindGuard and Surpass.
The Stream® product developed by Perflow is a mechanical thrombectomy device for acute stroke patients. It was CE certified in 2015 and has completed nearly 40 successful clinical operations in Germany and Israel. Stream? is like a fishing net that can automatically control the opening and closing. During the operation, the doctor introduces the patient's thigh blood vessels into the patient's skull, and then automatically opens the blood vessel to complete the grasping. 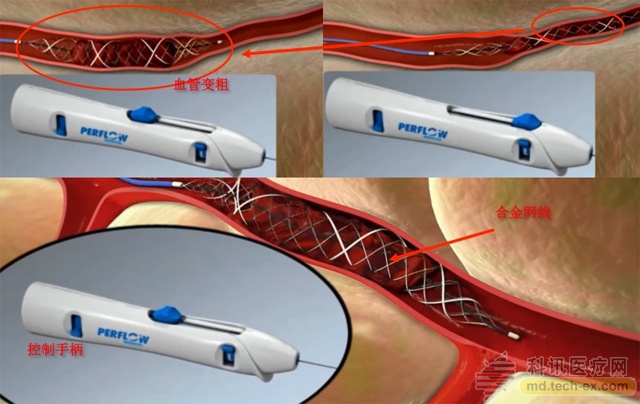
SreamTM intracranial thrombus capture device
At present, Medtronics, Johnson & Johnson and other companies take the lead in the market, and Perflow's innovative products have unique advantages. According to Chen Tingliang, the investment director of China-Israel Fund, unlike other laser cutting technology used in other products, Stream? uses bio-machining technology, which can realize flexible and stable geometric mesh, which is highly controllable. Through the unique development technology, the whole operation of the operation operation is monitored in real time to improve the rate of thrombus capture and reduce the risk of secondary thrombosis caused by emboli in the process of thrombectomy, and improve the cure rate and the effect.
It is understood that Perflow has already approved relevant qualifications for this product in China, the United States and other countries, and may obtain CFDA certification in the next 2-3 years to enter the Chinese market.
In addition, Perflow, a mechanical treatment device for brain hemangioma and giant brain hemangioma, is also under development.
Source: 36æ°ª
Lactobacillus Reuteri,Lactobacillus Reuteri Probiotic,Lactobacillus Reuteri Products,Lactobacillus Reuteri Supplements
Biodep Biotechnology Co. ,Ltd. , https://www.mbioda.com